Probiotics for the common cold: L. plantarum HEAL9 and L. paracasei 8700:2 Two meta-analyses have demonstrated that probiotics decreased the incidence and length of the common colds, or upper respiratory tract infections (URTIs).1,2 Over the last several years, mounting evidence suggests that a particular combination of specific probiotic strains, Lactobacillus (recently renamed Lactiplantibacillus) plantarum HEAL9 and Lactobacillus (recently renamed Lacticaseibacillus) paracasei 8700:2, provides larger benefit than the 8% relative risk reduction for infection2 or the 1.89-day decrease in cold duration.1
Cover art: Model of potential probiotic-mediated immune response in upper respiratory tract infections. Probiotic interaction with dendritic cells in intestine, dendritic cell migration to mesenteric lymph nodes to activate T cells, activated T cell migration through lymph and blood leading to activated T cells in the lungs. Adapted from Samuelson DR et al. Front Microbiol. 2015;6:1085.
The immune response in the common cold
Human coronaviruses, adenoviruses, and over a hundred rhinoviruses are the main viruses associated with causing the common cold. The detrimental effects of the common cold arise not from the infection by the virus itself but rather from the immune response. Immune cells known as polymorphonuclear leukocytes (PMN) increase in population around the infected nasal epithelial cells about one to two days after infection. PMN are activated to destroy harmful organisms to fight infections. Around the same time, symptoms start to occur. These symptoms include sore throat, coughing, and sneezing due to yellow or white mucus developing in the nasal passages, with additional symptoms such as fatigue and headaches also possible.
Selection of L. paracasei 8700:2 and L. plantarum HEAL9
A combination of lactobacilli strains in clinical trials showed reduction in the frequency, intensity, and duration of common cold symptoms: 1 billion colony forming units per day of equal numbers of L. plantarum HEAL9 (DSM 15312) and L. paracasei 8700:2 (DSM 13434).4–7 Prior to this work, preclinical and clinical evidence of immunoregulatory activity in the intestine suggested that these bacteria would benefit immune health as probiotics. Both L. plantarum and L. paracasei species frequently occur in the human gastrointestinal mucosa and have been shown to adhere to intestinal cells in vitro.8 L. paracasei 8700:2 has been shown in vitro to possess strong antagonistic properties against Salmonella enterica susbsp. enterica, as well as more intermediate antagonistic activity against Helicobacter pylori.9 In vivo studies also demonstrate reduced viable count of Enterobacteriaceae (a family of pathogens that includes disease-causing bacteria) in the colon, as well as reduced translocation (passage of viable bacteria through the epithelial mucosa into lymph nodes and other tissues).10,11 Additionally, either L. plantarum HEAL9 (DSM 15312) or L. paracasei 8700:2 (DSM 13434) alone inhibited and slowed the appearance of signs of a preclinical model of multiple sclerosis (called experimental autoimmune encephalomyelitis).12 Specifically, each strain individually reduced inflammation in autoreactive T cells and released antiinflammatory cytokines including IL-10 in spleen and mesenteric lymph nodes as well as reduced the autoreactive T cells in the central nervous system.12 This preclinical research suggested immunomodulatory activity of resident gastrointestinal strains and supports the immunological benefits observed in clinical studies.
Research highlights
- 1 billion colony-forming units (CFU) of the combination L. plantarum HEAL9 and L. paracasei 8700:2 supported immune defense in four double-blind, randomized, placebo-controlled clinical trials on the common cold
- This combination Lactobacillus (recently renamed Lactiplantibacillus) plantarum HEAL9 and Lactobacillus (recently reclassified as Lacticaseibacillus) paracasei 8700:2 has shown benefit for both children and adults
- Benefits included reducing the incidence of colds, fewer sick days, reduction in symptoms including runny nose, and lower use of accompanying medications
- This combination supports a balanced immune response, primes the immune system, and has been shown to survive through the gastrointestinal tract
- Additional research supports reduction in inflammation of stressed individuals (L. plantarum HEAL9)
Potential mechanisms of action
Clinical evidence suggests that these two strains modulate T cells and inflammatory markers for a balanced immune response. In a blind, placebo-controlled trial, L. paracasei 8700:2 was compared to five other lactobacilli strains in separate groups of randomly assigned subjects and demonstrated the following increases in antiviral activity:13
• An increase in the expression of the T cell memory marker CD45RO on CD8+ T cells (p=0.11). Memory CD8+ T cells can respond more quickly and effectively to viral infections.
• Increase in phagocytic activity of granulocytes, also called PMN (p=0.06). These white blood cells destroy potentially harmful microorganisms.
• Expansion of natural killer T (NKT) cell population (p=0.06). Stimulation of NKT cells suggests enhanced innate immune defense against viral infections. Natural killer (NK) cells are involved in the body’s first line of defense against viral infections by consuming infected cells and signaling to macrophages to phagocytose pathogens.14
These two strains have shown immune-modulating activity in two noncold-related randomized, double-blind placebo-controlled clinical trials. For example, a six-month study using 10 billion CFU of the combination in children at genetic risk for celiac disease demonstrated changes in T cells.15 The placebo group experienced an increase of certain memory and effector T cells that have been observed to be elevated in untreated celiac disease patients; these cells remained unchanged in the probiotic consumers, suggesting that the placebo group was responding to regulate inflammation in the presence of dietary gluten antigens while the probiotic group may not have needed to regulate intestinal inflammation due to the lack of immune response. The significant differences in peripheral immune responses between the placebo and probiotic group suggested that the probiotic combination may help support a healthy immune response. Another example involved the use of 10 billion CFU of the L. plantarum HEAL9 on people who were given a stress test, showing that the probiotic-consuming group had decreased levels of inflammatory markers during the stress test.16 These clinical examples suggest additional mechanisms of immune modulation via T-cells and inflammatory markers.
Clinical trials of L. paracasei 8700:2 and L. plantarum HEAL9 in colds The 1 billion CFU combination of L. paracasei 8700:2 and L. plantarum HEAL9 benefited adults (3) and children (1) in a total of four clinical trials. In summary, effects observed included a reduced incidence of the common cold, fewer sick days, milder common colds, and reduced use of concomitant medications. A double-blind, placebo-controlled, multicenter study randomized 272 healthy subjects (aged 18-65, mean 43.7) into to two groups receiving either placebo or probiotic—1 billion CFU of the lactobacilli combination—for 12 weeks during the common cold season. Subjects were restricted from any probiotic consumption prior to and during the intervention as well as influenza vaccination within the last year. Subjects recorded daily logs of health status including severity of common cold symptoms: nasal (runny or stuffed nose, yellow or bloody secretions, sneezing), pharyngeal (scratchy or sore throat, hoarseness), bronchial (cough, secretion, yellow secretion), headache, myalgia, conjunctivitis, earache, sinusitis, fatigue, fever, nausea, and loss of appetite. The start of a common cold episode was defined as the occurrence of a total symptom score of 2 or more and defined as over when the sickness score was less than 1 for nasal, pharyngeal, or bronchial symptoms.
Highlights of results:
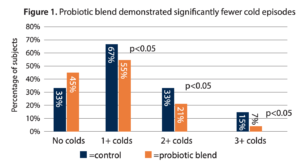
- Fewer cold episodes. The incidence of one or more cold episodes was significantly reduced from 67% in the placebo arm to 55% in the probiotic arm (p=0.043). The difference was even more pronounced for two or more episodes, with a significant reduction from 33% to 21% (p=0.0024, probiotic compared to control) (Figure 1).
- Delayed onset of episodes. The mean occurrence of the first cold was significantly delayed in probiotic subjects to 30.5 days, compared to 21.9 days for control (p=0.038). A similar trend was observed for episodes that followed.
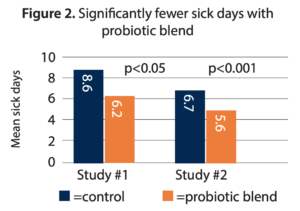
- Reduced number of days sick during the intervention. The probiotic arm demonstrated a significant reduction in sick days at a mean of 6.2, compared to a mean of 8.6 in the control group (p<0.05) (Figure 2) over the 12-week intervention.
- Reduced symptom severity. In subjects with one or more cold episodes, the probiotic arm demonstrated lower total and individual scores for pharyngeal, nasal, and bronchial symptoms, not statistically significant when compared to placebo except for the score for pharyngeal symptoms (p=0.027). For subjects experiencing a second episode, the total symptoms score was significantly lower in the probiotic arm compared to the control (p=0.031).
- Probiotic detection in feces. Fecal samples were taken after the two-week run-in phase and compared to samples after 2 and 12 weeks of intervention. The levels of L. plantarum and L. paracasei bacteria increased over the course of the probiotic intervention, significantly at 2 weeks and 12 weeks compared to baseline measures.
A second clinical study of similar design was later conducted with 310 subjects with greater risk of common cold (aged 18-88 years, mean 46.0). To participate, subjects were to have experienced at least two episodes of the common cold within the previous six months. Subjects kept diaries and scored 13 cold symptoms on a scale of 0 to 3. Highlights of this 12-week study include:
- Reduced duration of episodes. The mean number of sick days was significantly lower at 5.6 in the probiotic arm compared to 6.7 days for control (p<0.001) (Figure 2).
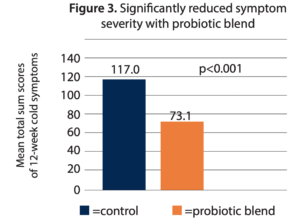
- Reduced total symptom severity. The total mean score of cold symptoms over 12 weeks was significantly lower in the probiotic arm at 73.1, compared to 117.0 for the placebo arm (p<0.001) (Figure 3). Most of the individual symptom scores were also significantly lower compared to control.
- Reduced day-to-day symptom severity. An analysis of a seven-day cold episode further demonstrates faster recovery and reduced symptom intensity. At episode onset both arms showed similar mean total symptom scores, but the probiotic arm showed a reduction in total symptoms in all the following days—which was statistically significant days 4-7 (Figure 4).
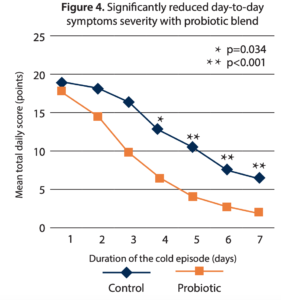
While the probiotic arm showed a slightly lower incidence of cold episodes and a slightly delayed onset of first illness when compared to control, these results were not statistically significant. The researchers attribute this unexpected finding to this study’s population of frequent cold sufferers. However, the reduction in severity of symptoms this study not only confirmed the findings of the first study with the lactobacilli combination, but they were also more pronounced.
In another 12-week, double-blind, placebo-controlled, randomized study using the same combination, 131 children attending day care were monitored by caregivers regarding the children’s cold symptoms using a validated scale, days absent from day care, and usage of medications, the interventional product, and compliance to the protocol. This study planned to enroll 320 healthy children; however, the clinical research organization shut down, which reduced the final number. Reduction in mean total severity score per day in subjects with at least one URTI would have been significant if the full enrollment number reflected the same pattern observed in the smaller group studied. Major findings included the following:7
- Reduced duration of third episode. Fewer children had three colds during the 12-week intervention in the probiotic group (8 v. 15). Although the first and second episodes did not appear to differ significantly between groups, the third episode, if experienced, was significantly shorter in the probiotic group (4.0 ± 2.2 days and 6.6 ± 2.8 days p=0.023) reported by 8 children in the probiotic group v. 15 children in the placebo group.
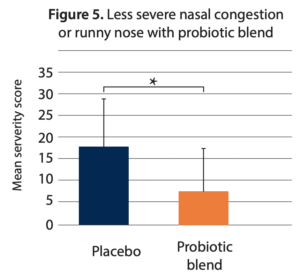
- Reduced severity of nasal congestion/runny nose. The mean severity score of “nasal congestion/runny nose” for the whole study period was 7.5 ± 9.7 in the probiotic group and 13.9 ± 15.2 in the placebo group (p = 0.024) (Figure 5).
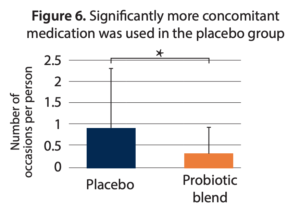
- Reduced usage of medications. The mean number of medications used was 0.3 ± 0.65 in the probiotic group compared to 0.9 ± 1.4 in the placebo group (p = 0.018) (Figure 6).
The largest double-blind, placebo-controlled trial on this combination to date included 14 sites and randomized 898 adults who had four colds in the last 12 months, with ages spanning 18-70 years over three cold seasons.6
- Reduction in the incidence of colds. A significantly lower incidence of colds was identified in the probiotic group compared to the placebo group during the first cold season, with 50/123 (40.7%) and 67/125 (53.6%) participants, respectively, reporting at least one cold (p=0.043).
- Reduction in daily symptoms. There was a significant difference in mean daily Wisconsin Upper Respiratory Symptom Survey−21 (WURSS-21) score (1.44 ± 2.48) compared to placebo (2.73 ± 4.31; p=0.019) during the first cold season but not in the other two seasons. Nasal, pharyngeal, and bronchial symptoms were less severe in the probiotic group compared to the placebo group (p=0.028) in the cold season of 2013–2014.
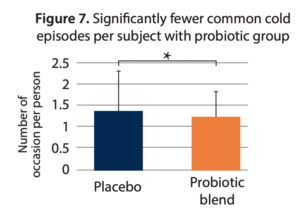
- Reduced recurrence of colds. Significantly fewer colds were experienced in the probiotic group (mean of 1.24 colds) as compared to the placebo group (mean of 1.36 colds; p=0.044) for subjects reporting at least one cold. The incidence of recurring colds was 30% lower (20.8% vs. 29.8%, respectively; p=0.055) illustrated as significantly fewer common cold episodes per subject overall (Figure 7).
- Reduced use of analgesics. The use of analgesics was 18% lower (26.3% vs. 32%, respectively; p=0.07).
Summary
In four large-scale, randomized, placebo-controlled studies, daily administration of a combination of L. plantarum HEAL9 and L. paracasei 8700:2 showed benefits for adults or children having colds. Significant reductions in the intensity of total cold symptoms and key individual symptoms and reduction in sick days were observed. Furthermore, in the trial over multiple seasons, there was a reduction in the recurrence of colds. Additional clinical trials suggest that mechanisms of supporting these immune responses include modulation of T cells, inflammatory markers, and balancing the immune response. This probiotic combination may provide a useful therapeutic application in clinical practice for reducing the severity, duration, and frequency of cold-like illnesses.


